Docetaxel and Doxorubicin Codelivery by Nanocarriers for Synergistic Treatment of Prostate Cancer
Prostate cancer (PCA) remains a significant health challenge worldwide, necessitating innovative therapeutic strategies. Combination chemotherapy, utilizing multiple chemotherapeutic agents, has shown promise in enhancing treatment efficacy. However, differences in the pharmacokinetics of these drugs often hinder their synergistic potential. To address this, our study explores the co-delivery of docetaxel (DOC) and doxorubicin (DOX) using nanocarriers, aiming to optimize their combined therapeutic effects against PCA.
Design and Characterization of DDC Nanoparticles
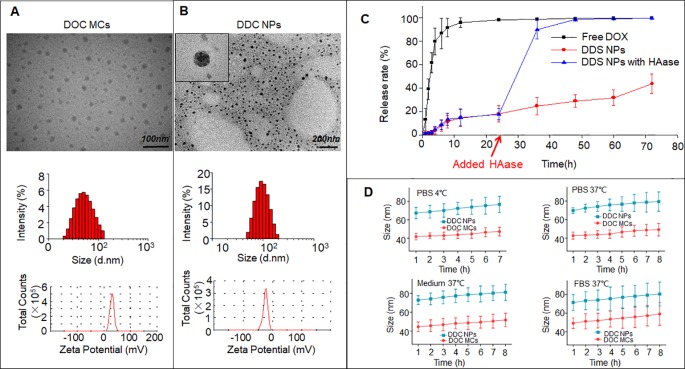
We developed DOC and DOX co-delivery nanoparticles (DDC NPs) through a self-assembly process involving hyaluronic acid (HA) and cationic amphipathic starch (CSaSt). Transmission electron microscopy revealed that these nanoparticles are spherical with rough surfaces, averaging 68.4 ± 7.1 nm in size and possessing a zeta potential of -22.8 ± 2.2 mV. The encapsulation efficiencies for DOC and DOX were 96.1 ± 2.3% and 91.4 ± 3.7%, respectively, with a total drug loading of 9.1 ± 1.7%. Importantly, the DOC to DOX ratio within the nanoparticles was approximately 1:400, aligning with the optimal synergistic proportions identified in our study.
In Vitro Efficacy
The DDC NPs demonstrated sustained and enzymatic drug release while maintaining stability in phosphate-buffered saline (PBS), culture medium, and serum. In vitro assays using human PCA cell lines (PC-3, DU-145, and LNCap) indicated that DDC NPs were as effective as the dual drug combination in inducing cytotoxicity, inhibiting cell migration, and promoting apoptosis. The internalization of DDC NPs by PCA cells was facilitated by the interaction between HA and the CD44 receptor, ensuring efficient delivery and release of the therapeutic agents within the target cells.
In Vivo Therapeutic Outcomes
In vivo studies utilizing PCA cell xenograft mouse models revealed that DDC NPs significantly enhanced the accumulation of DOC and DOX in tumor tissues while reducing nonspecific distribution in normal organs. This targeted delivery resulted in a notable improvement in therapeutic efficacy, with DDC NPs promoting greater tumor suppression compared to free drug combinations. Furthermore, acute toxicity and hemolytic assays confirmed the low toxicity profile of DDC NPs, underscoring their potential for safe clinical application.
Conclusion
Our findings suggest that the co-delivery of DOC and DOX via HA-CSaSt-based nanoparticles offers a promising approach for synergistic chemotherapy in prostate cancer treatment. The DDC NPs not only optimize the therapeutic ratio of the drugs but also enhance their delivery to tumor sites while minimizing systemic toxicity. This nanocarrier system holds significant potential for advancing PCA chemotherapy and warrants further clinical investigation.